Leveraging the powerful translational science linking the Cothera’s proprietary i-CR® technology platform and clinical patient drug response, Cothera Bioscience has developed several innovative anticancer candidates in the field of synthetic lethality, protein degradation and immunotherapy. Cothera Bioscience has applied for multiple international patents covering the drug candidates as well as the i-CR® technology platform.
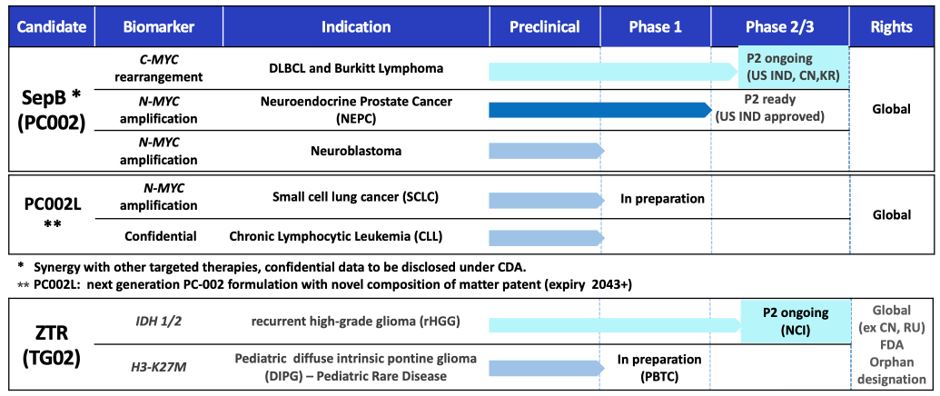
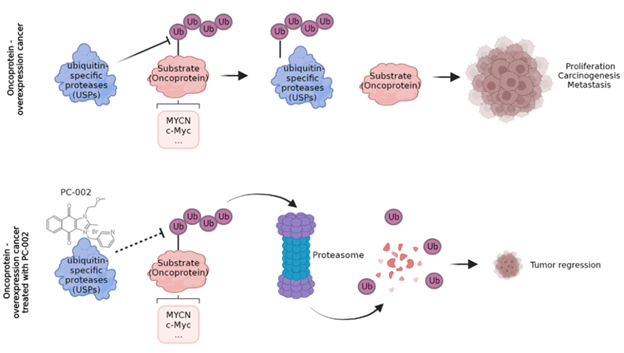
Sepantronium Bromide (PC-002)
Cothera’s lead program PC-002 (also known as YM155, sepantronium bromide) is a first-in-class small molecule drug inhibitor of DUB, an enzyme family which removes ubiquitin from cellular proteins otherwise destined for degradation. With more than 50% of human cancers showing increased expression, Myc is regarded as one of the most important yet “undruggable” cancer targets. By inhibiting DUB that stabilizes the Myc protein in cancer cells, PC-002 selectively induces Myc protein degradation and cell apoptosis in Myc-dependent tumors. PC-002 may potentially target multiple cancer indications involving Myc dysregulation.
A paper1 describing PC-002’s mechanism-of-action has been accepted for publication on European Journal of Pharmaceutical Science.
1. Li et al. YM155 Inhibits Neuroblastoma Growth through Degradation of MYCN: A New Role as a USP7 Inhibitor, European Journal of Pharmaceutical Sciences (2022) doi: https://doi.org/10.1016/j.ejps.2022.106343
PC-002 is currently in Phase 2 studies for multiple indications and has demonstrated initial efficacy as well as safety in an ongoing monotherapy trial in relapsed and refractory high-grade B-cell lymphoma and Burkitt lymphoma patients with myc rearrangement (NCT05263583). Cothera presented the data at the European Hematology Association (EHA) 2023 Congress (June 2023) as well as at the 65th American Society of Hematology (ASH) annual meeting (December 2023).
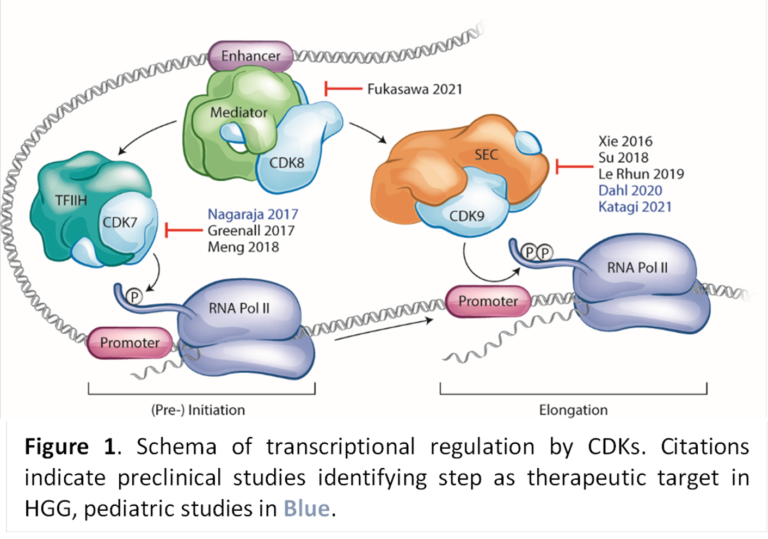
Zotiraciclib (ZTR/TG02)
The second compound the company is developing is zotiraciclib (ZTR/TG02). ZTR is a potent oral CDK9 inhibitor that crosses the blood-brain barrier and degrades short-lived, anti-apoptotic oncogene proteins. The compound is being developed for the treatment of high-grade glioma (HGG) and diffuse intrinsic pontine glioma (DIPG), both brain cancers with high unmet medical need. ZTR has successfully completed a Phase 1b clinical trial sponsored and conducted by the US National Cancer Institute (NCI) that confirmed the safety and initially demonstrated effectiveness of ZTR in combination with temozolomide (TMZ) in patients with recurrent high-grade glioma (Clin Cancer Res 2021 Jun 15;27(12):3298-3306). Recently the compound also demonstrated initial efficacy as well as safety in an on-going monotherapy trial targeting IDH-mutant high-grade glioma sponsored by NCI (NCT05588141).