i-CR® Drug Discovery Platform
i-CR® drug screening platform paves the way for more anti-tumor cocktail therapy discovery.
The Only i-CR® Drug Screening Platform with Proprietary IP in the World
- i-CR® is the only platform for high-throughput drug screening on primary cancer cells with proprietary IP
- Effective drug and drug combination can be identified with ultra-high sensitivity and detection window
- The platform has been clinically validated with excellent performance
Cothera Bioscience developed a proprietary i-CR® technology platform, a powerful method for individualized drug screening and new drug development. The platform combines a high content screening system with conditional reprogramming of primary tumor cells, which enables efficient and homogeneous expansion of primary patient-derived tumor cells in vitro while preserving the tumor’s heterogeneity. Through collaboration with leading oncology medical centers, Cothera Bioscience has conducted prospective clinical trials and demonstrated that the i-CR® system can effectively predict the actual clinical response in patients. This gives us the opportunity to significantly improve the clinical efficacy and success rate in anticancer drug development (Transl Oncol. 2021 Jan;14(1):100935).
Leveraging the powerful translational science linking the i-CR® technology platform and clinical patient drug response, Cothera Bioscience has developed a number of innovative anticancer candidates based on the mechanisms of synthetic lethality, protein degradation and immunotherapy. Cothera Bioscience has applied for multiple international patents covering the drug candidates as well as the i-CR® technology platform.
Core Products
Cothera Bioscience is focusing on developing therapeutics for previous undruggable oncology targets with high unmet medical needs. Current pipeline includes three clinical stage programs targeting Myc and KRAS.
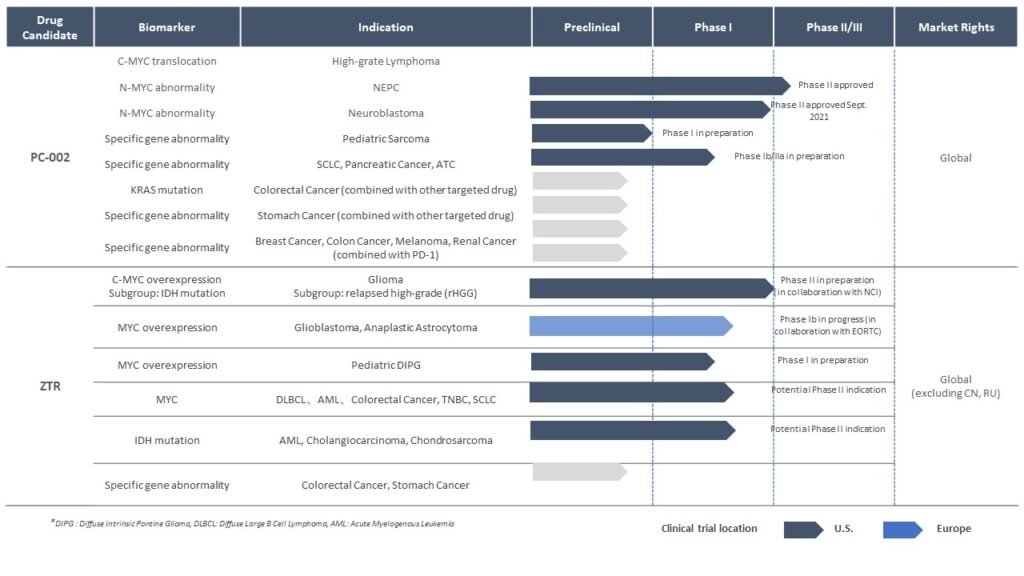
Sepantronium Bromide (PC-002)
Currently in Phase 2 studies for multiple indications, Cothera’s lead program PC-002 is a first-in-class small molecule drug targeting Myc- mutated tumors. With more than 50% of human cancers showing increased expression, Myc is regarded as one of the most important yet “undruggable” cancer targets. With a unique mechanism of action, PC-002 selectively induces Myc protein degradation and cell apoptosis in Myc-dependent tumors, potentially targeting multiple indications in cancers involving Myc dysregulation.
Zotiraciclib (TG02)
The second compound the company is pursuing for treating Myc-dysregulated tumors is zotiraciclib (ZTR/TG02). ZTR is a potent oral CDK9 inhibitor that crosses the blood-brain barrier and down-regulates short-lived, anti-apoptotic oncogene proteins such as Myc. The compound is being developed for the treatment of high-grade glioma (HGG, Phase 2) and diffuse intrinsic pontine glioma (DIPG, Phase 1/2), both brain cancers characterized by Myc overexpression. ZTR has successfully completed a Phase 1b clinical trial sponsored and conducted by the US National Cancer Institute (NCI) to confirm the safety and demonstrate initial efficacy of ZTR in combination with temozolomide (TMZ) in patients with recurrent high-grade glioma (Clin Cancer Res 2021 Jun 15;27(12):3298-3306).
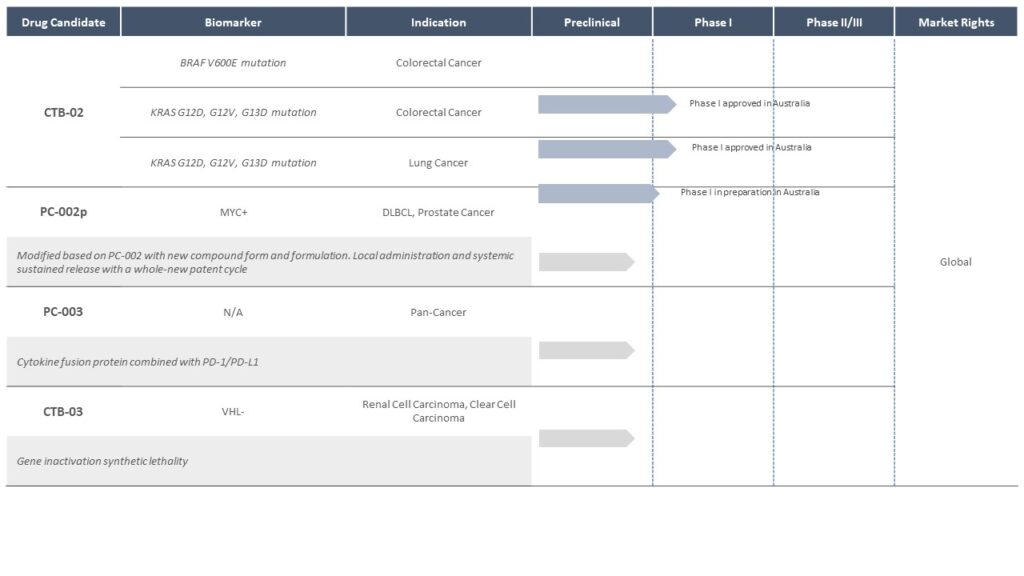
CTB-02
The company’s third pipeline product, CTB-02, targets colon cancer and non-small cell lung cancer with pan-KRAS mutations. There are currently no approved targeted therapies for KRAS-mutant colorectal cancer, resulting in huge clinical unmet needs. In CDX and PDX animal experiments, CBT-02 demonstrated powerful inhibitory effect not only on KRAS G12C-mutated colorectal cancer, but also on cancers with other KRAS mutations. CTB-02 is currently in Phase 1/2 clinical trial in Australia.
Product Pipeline