Zotiraciclib (TG-02)
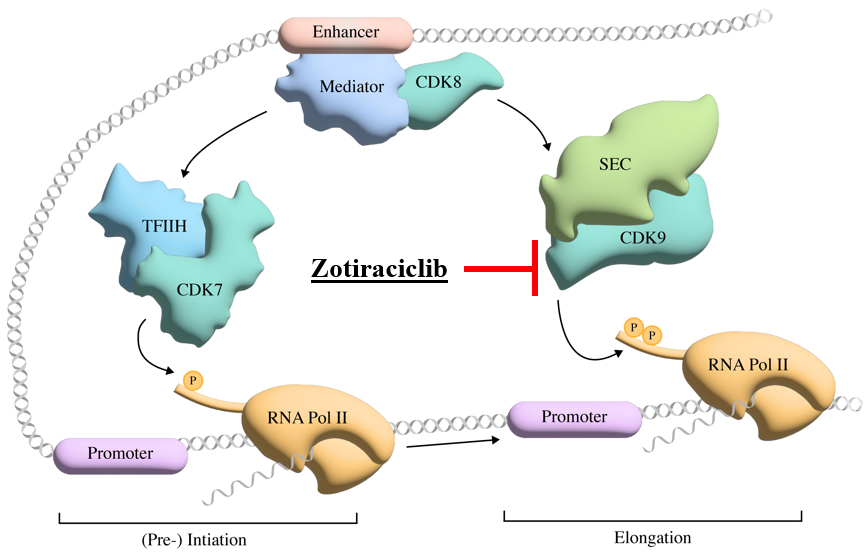
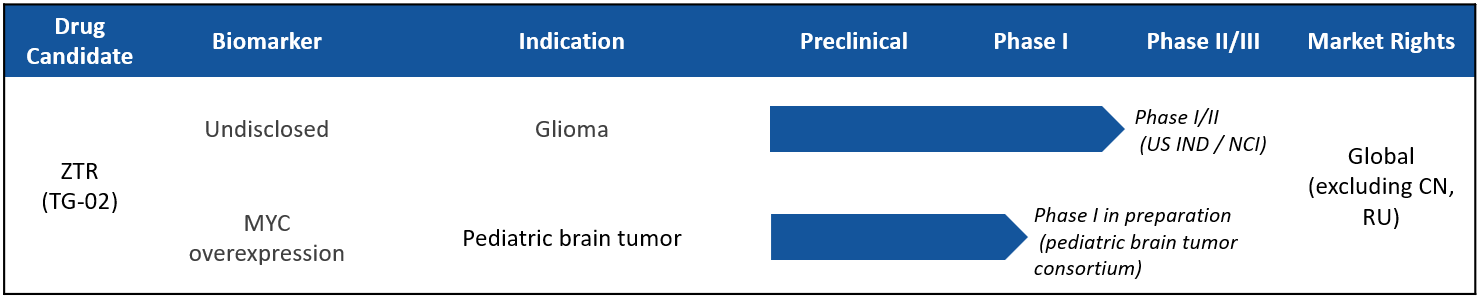
The second compound Cothera is pursuing for treating Myc-dysregulated tumors is zotiraciclib (TG-02). TG-02 is a potent oral CDK9 inhibitor that crosses the blood-brain barrier and down-regulates short-lived, anti-apoptotic oncogene proteins such as Myc. TG-02 is being developed for the treatment of high-grade glioma and diffuse intrinsic pontine glioma both brain cancers characterized by Myc overexpression. ZTR has successfully completed a Phase 1b clinical trial sponsored and conducted by the US National Cancer Institute (NCI) to confirm the safety and demonstrate initial efficacy of ZTR in combination with temozolomide (TMZ) in patients with recurrent high-grade glioma (Clin Cancer Res 2021 Jun 15;27(12):3298-3306).
Cothera is currently preparing to initiate a Phase 1/2 study with NCI for recurrent high-grade glioma with IDH mutations. Cothera is also preparing to initiate a Phase 1 study for pediatric brain tumor.